
About the Korlym pivotal trial
Patients receiving Korlym showed significant decreases in their mean body weight, and decreased their symptoms of depression1
*Because of the variability in clinical presentation and variability of response in this open label trial, it is uncertain whether these changes could be ascribed to the effects of Korlym.2
Reach for Korlym to fight back with significant improvements in glucose control
60% of patients achieved a ≥25% reduction from baseline in AUCglucose at week 24/ET1*†
Significant improvements in oGTT were seen as early as week 6 and continued through week 24.1

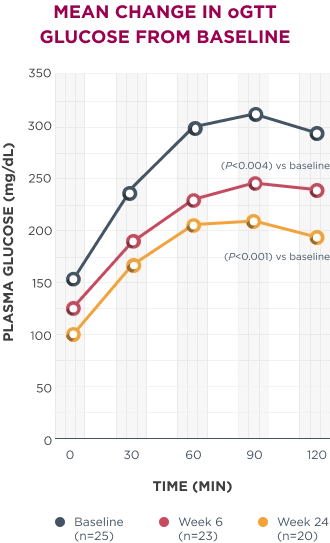
AUC, area under the curve; ET, early termination; HbA1c, hemoglobin A1c; oGTT, oral glucose tolerance test.
*In patients with Cushing syndrome and either T2D or impaired glucose tolerance.1
†Those with a ≥25% decrease in AUCglucose from baseline to week 24 were considered responders. This primary efficacy endpoint was considered met if ≥20% of patients were responders.1
Early and significant improvements in AUCinsulin
Improvements in AUCinsulin were seen as early as week 6 and continued through week 241


Error bars in the graph are SD. A combined cohort of patients not treated with insulin.
*P<0.001 vs baseline.
†P=0.002 vs baseline.
Reach for Korlym to fight back with reductions in HbA1c and T2D medications
Significant 1.1% reduction in HbA1c at week 241,3*


A majority of patients with HbA1c >7% at baseline reached their HbA1c goals.1
75% of patients achieved HbA1c goal <7% (n=9/12)
50% of patients achieved HbA1c goal <6% (n=6/12)
A significant 1.9% mean reduction was observed by week 24/ET (n=12).3
*In patients with Cushing syndrome and either T2D or impaired glucose tolerance.
Reductions in T2D medications
Some patients were able to reduce their T2D medications with Korlym:
of patients were able to
reduce their daily T2D
medication (n=7/15)2
of patients were able
to reduce their daily insulin by at
least half (n=5/12)1
Reach for Korlym to fight back with significant reductions in weight and depression
Patients treated with Korlym experienced a significant 5.7% (6.3 kg) reduction in mean body weight at week 24/ET1*†‡


Most patients experienced reductions in body weight1:
of patients reduced
body weight by ≥5%
(n=24/46)
of patients reduced
body weight by ≥10%
(n=12/46)
Because of the variability in clinical presentation and variability of response in the open-label trial, it is uncertain whether the change in body weight could be ascribed to the effects of Korlym.2
†In the modified intent-to-treat population, defined as patients who received >30 days of study medication.1
‡Mean baseline BMI was 35.7 kg/m2 in the intent-to-treat population.1
BMI, body mass index.
Patients experienced significant improvements in depression*
Nearly 60% of patients with mild-to-severe depression improved to minimal depression by week 24 (n=14/24)1,4,5


*Because of the variability in clinical presentation and variability of response in the open-label trial, it is uncertain whether the change in psychiatric symptoms could be ascribed to the effects of Korlym.2
BDI-II, Beck Depression Inventory®-II.